Abstract
Our long-term goal is to create a vehicle for transplanting skeletal muscle progenitor cells to promote the enlargement of natural bypass arteries in patients with peripheral arterial occlusive disease. For this project, hydrogel-based cell vehicles were made from pluronic F-127 and sodium alginate, and were extruded using a 3D bioprinter. Cell viability and distribution were determined in vitro, and in vivo pilot experiments were conducted to measure natural bypass artery diameter in preclinical murine models.
Our Team
Below is a brief introduction to the students that worked on the 2021 Bioprinting Hydrogel for Cell Therapy Delivery SURP project.

Rayana Gutierrez
Rayana Gutierrez is a 3rd year Biomedical Engineering undergraduate student at California Polytechnic State University, San Luis Obispo. Rayana was responsible for performing murine surgical procedures, immunofluorescent staining, and collecting arterial morphometry data. She worked in collaboration with Tori Barrington in synthesizing the bioink and printing with the BioBot. Rayana plans to continue the SURP project and conduct related studies in the MaVR throughout the rest of her time at Cal Poly. Besides research, Rayana is also involved in the Medical Design Club, and is the club’s Co-President for the current academic year.

Tori Barrington
Tori Barrington is a 4th year Biomedical Engineering undergraduate student at California Polytechnic State University, San Luis Obispo. Tori was responsible for myofiber isolation and cell culture and worked in collaboration with Rayana for bioink synthesis and bioprinting. During the school year, Tori will be continuing the research started in SURP along with working with faculty to augment BMED curriculum to reflect the current state of DEI in the biotech/medical industry. She hopes to continue her education at Cal Poly, pursuing a Master’s degree in the Regenerative Medicine Program.
Acknowledgements
We would like to acknowledge our advisor, Dr. Trevor Cardinal, the members of the Microcirculation and Vascular Regeneration (MaVR) lab, and the Cal Poly SURP coordinators. Their support and assistance was greatly appreciated and integral to the success of this project.
Our Project's Digital Poster
Introduction
- 200 million + people suffer from Peripheral Arterial Disease (PAD)
- Caused by plaque buildup in arteries leading to inadequate blood flow in limbs
- Treated with enlargement of natural bypasses via arteriogenesis
- Implanted myoblasts improve arteriogenesis
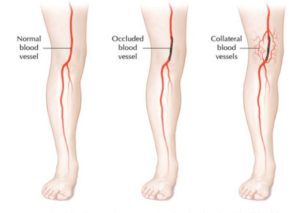 Figure 1. Collateral Blood Vessels [1]
Figure 1. Collateral Blood Vessels [1]
Cell Culture
- Hindlimb muscles excised from mice
- Myofibers isolated and placed in media
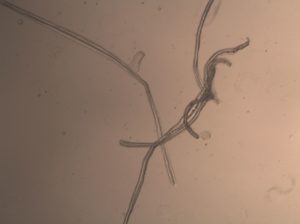 Figure 3. Isolated Myofibers
Figure 3. Isolated Myofibers
- Satellite cells migrate off myofibers and mature into myoblasts
- Factors added to discourage myoblast differentiation
 Figure 4. Myoblasts in Culture
Figure 4. Myoblasts in Culture
- Day 6-8 cells can be mixed into prepared bioink
Surgery
- Femoral artery ligation mimics arterial occlusion found in patients with PAD
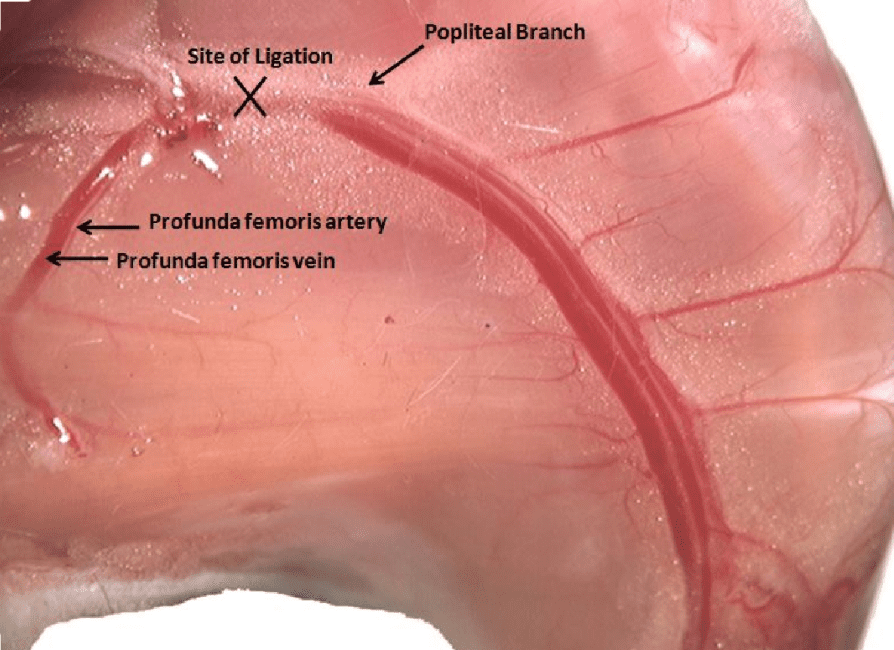
- Perfusion fixation clears the blood from the body and preserves the tissue
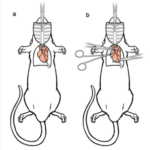
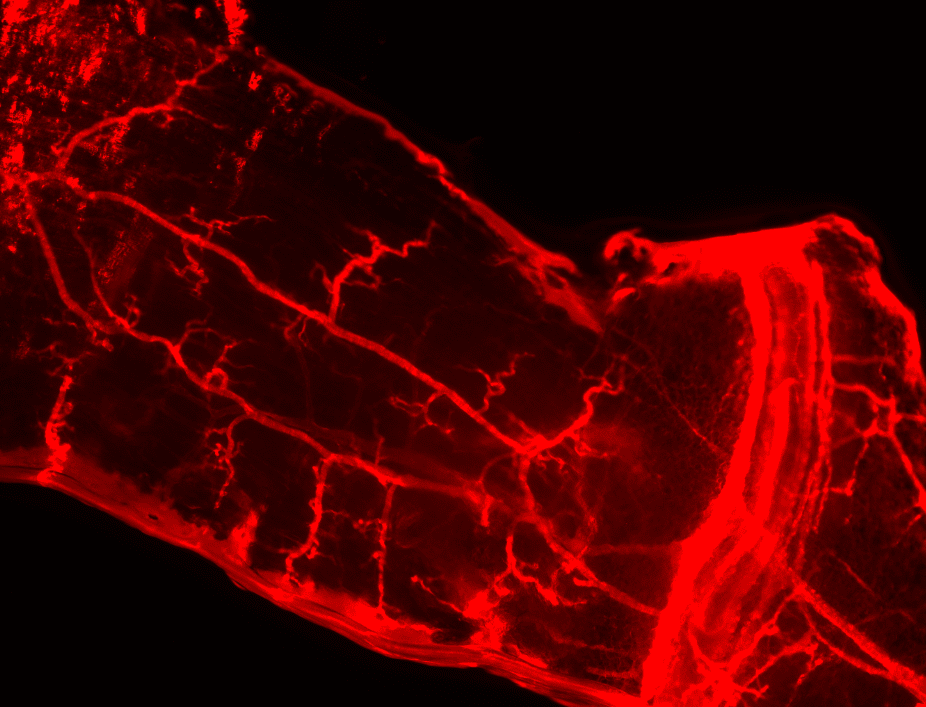
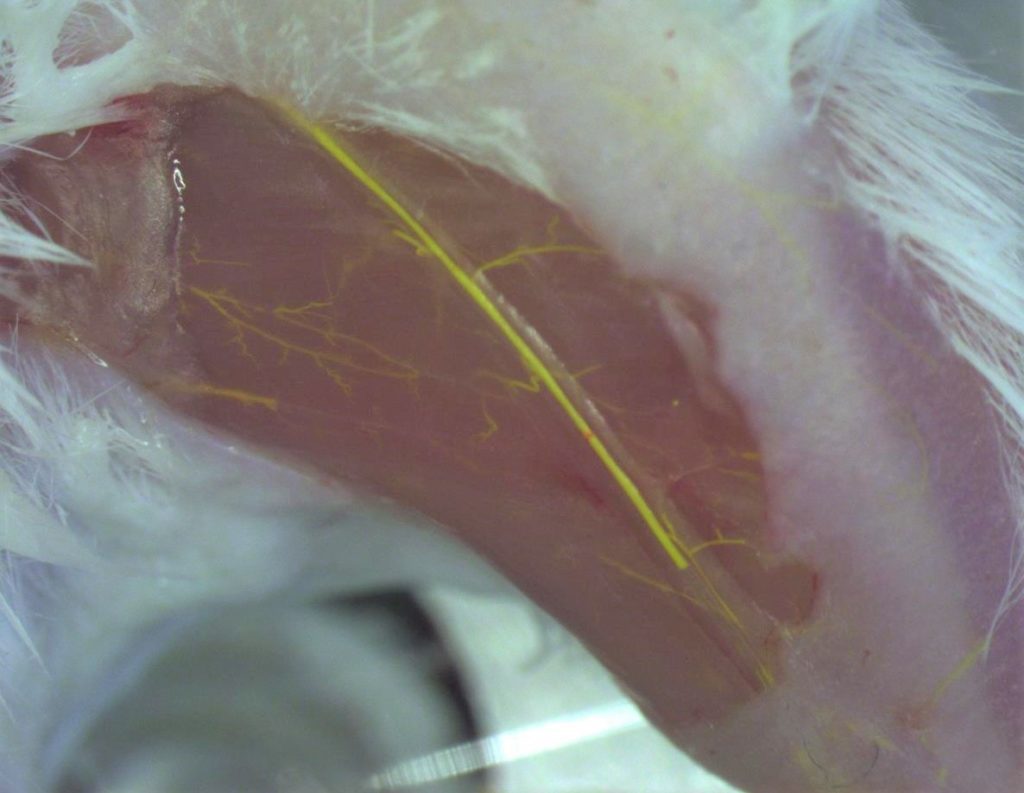
- Vascular casting and immunofluorescence staining performed to visualize collaterals and measure vessel diameter

Bioink Preparation
- Sodium Alginate (2.5% w/w) and Pluronic F127 (15% w/w) mixed with deionized water at 4℃ for ~4 hours
- Fluorescent fibroblasts cells printed at a density of 853,000 cells/mL ink
Bioprinting

- Transport vehicle made from Pluronic F-127 and sodium alginate demonstrate cell-responsive properties
- The BioBot is an extrusion-based bioprinter that is capable of printing hydrogels of various viscosity
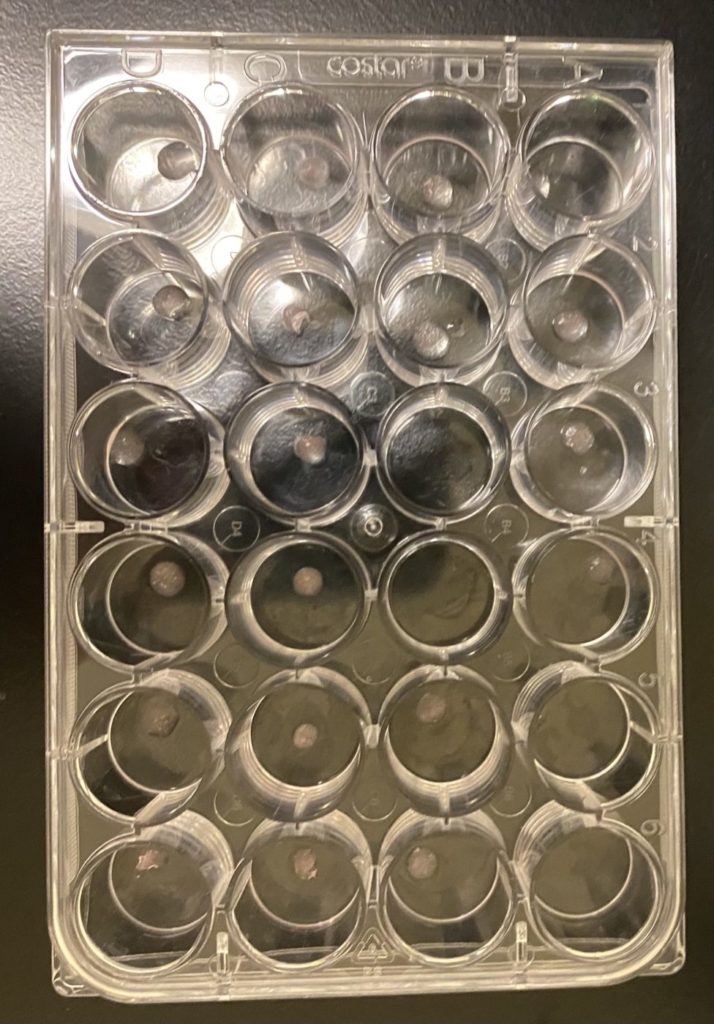
- Disk shaped constructs of 3mm diameter and 1 mm height printed at 10 PSI
- Crosslinked with CaCl2 solution
- Cells in transport vehicles stained and imaged to assess cell viability post-print
Project Workflow
- Myoblast Culture
- Myofibers isolated from murine hindlimb
- Myoblasts cultured from fibers
- Preliminary studies performed with fluorescent fibroblasts
- Bioink Preparation
- Sodium alginate
- Pluronic F127
- Cell suspension
- Bioprinting
- Print transport vehicles
- Crosslink
- Vehicle Implantation
- Implant vehicle under ligation site
- Collateral Vessel Assessment
- Perfusion fixation
- Vascular casting
Results
Construct Printed with Fluorescent Fibroblasts
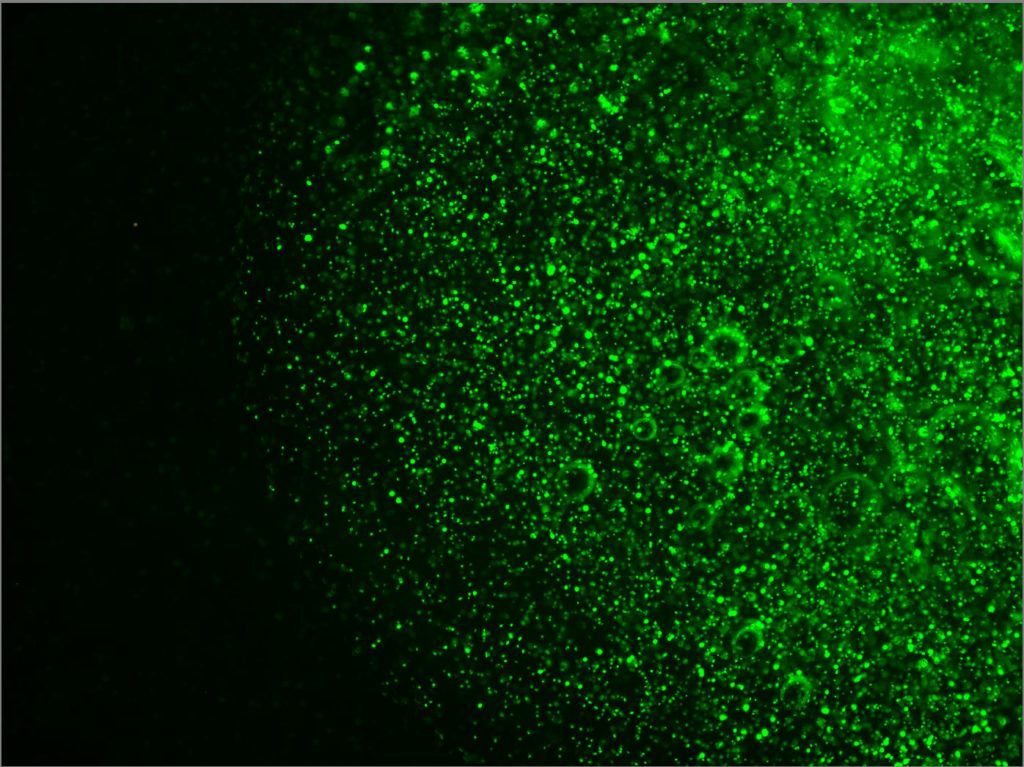
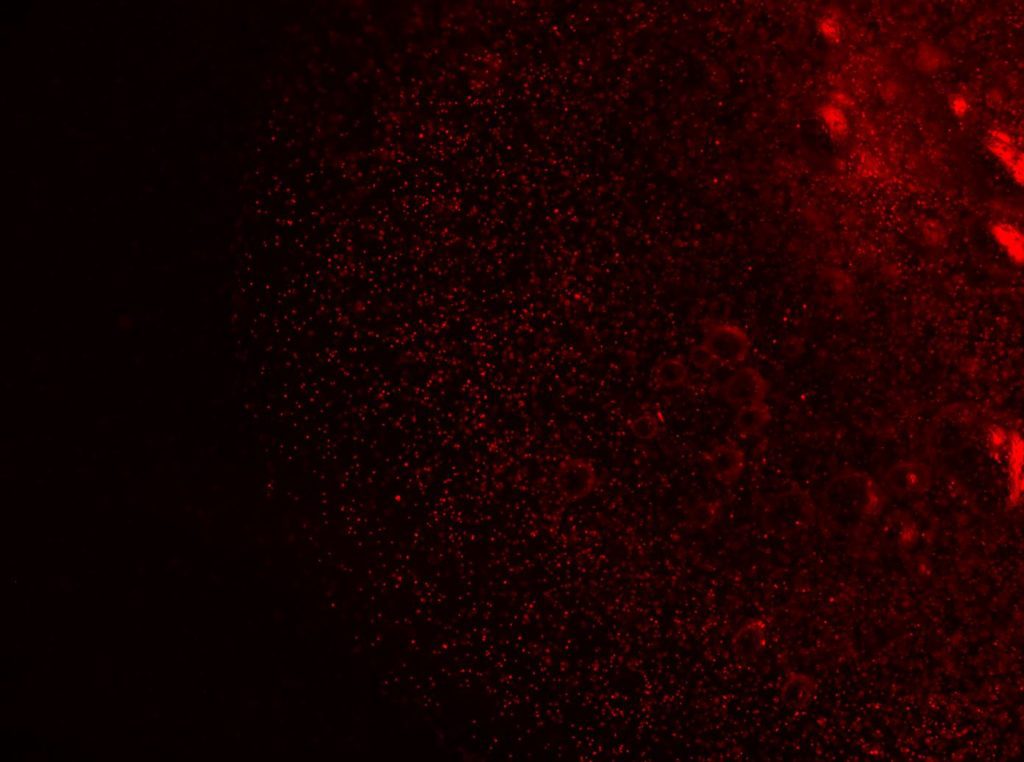
- 85.3% cell viability before cell solution mixed into bioink
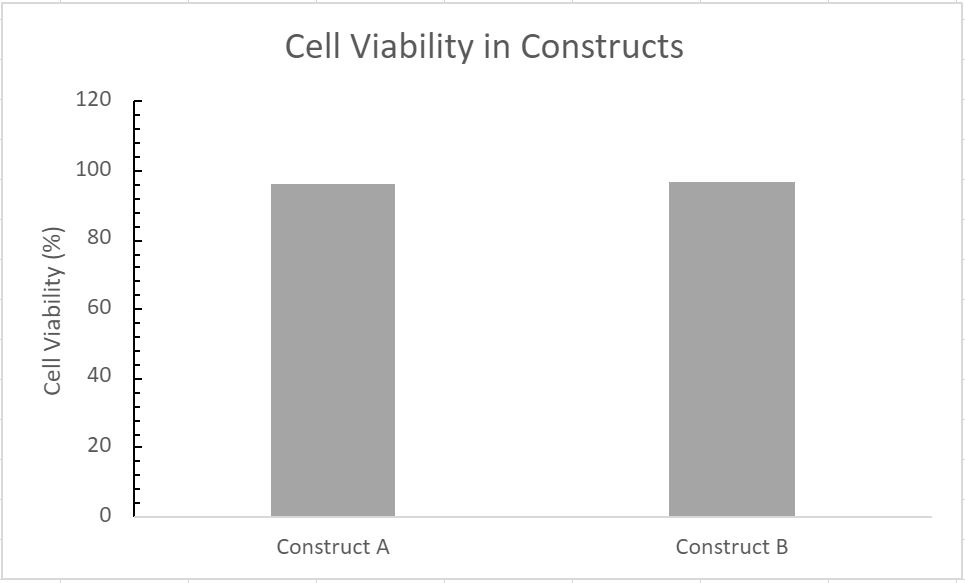
- 95.73% cell viability in Construct A
- 96.41% cell viability in Construct B
Future Work
- Utilize a confocal microscope to obtain a 3D visualization and quantification of distribution throughout the transplant vehicle
- Incorporate myoblasts into construct
- Conduct implantation study of cell delivery system
References
[1] Peripheral Artery Disease. Heart Health for Your Legs. (2014, March 01). Retrieved from https://healthletter.mayoclinic.com/issues/ march-2014/peripheral-artery-disease
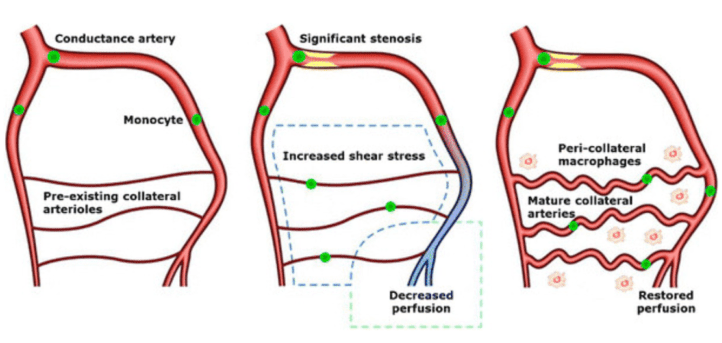
[2] Hendrikx, Geert & Vöö, Stefan & Bauwens, Matthias & Post, Mark & Mottaghy, Felix. (2016). SPECT and PET imaging of angiogenesis and arteriogenesis in pre-clinical models of myocardial ischemia and peripheral vascular disease. European Journal of Nuclear Medicine and Molecular Imaging. 43. 10.1007/s00259-016-3480-8.
[3] Gallagher, R. R.. (2012) The Impact of Outward Remodeling on Vasodilation in Skeletal Muscle Resistance Arteries. Retrieved from https://www.semanticscholar.org/paper/THE-IMPACT-OF-OUTWARD-REMODELING-ON-VASODILATION
[4] Gage, G. J., Kipke, D. R., Shain, W. (2012, July 30). Whole Animal Perfusion Fixation for Rodents. Retrieved from https://www.jove.com/t/3564/whole-animal-perfusion-fixation-for-rodents
[5] Collen, R. (2020, September). Development of Three-Dimensional Hydrogel Constructs for Cell Transplantation Using Extrusion-Based Bioprinting Technology

