Our Team
Danica Wong and Sydney Fultz-Waters worked on the SURP project, Development of Cost-Effective, Recyclable Nanosensors for Rapid Detection of Water Pathogens, over the course of the summer.

Danica Wong
Danica Wong is a second-year mechanical engineering major attending California Polytechnic State University, San Luis Obispo. She is currently a member of the Prototype Vehicles Laboratory (PROVE) at Cal Poly where she worked on various aspects of the vehicle including headlights, battery, and chassis. In this SURP project, she worked on testing possible candidate materials for colorimetric sensing, as well as determining methods for coating nanoparticle candidate materials with cationic polymers.

Sydney Fultz-Waters
Sydney Fultz-Waters is a third-year materials engineering major attending California Polytechnic State University, San Luis Obispo. Sydney worked on testing candidate materials and determining polymer coating methods for this SURP project. Some of her areas of academic interest include nanotechnology, electronic materials, and composites. Sydney is working part-time as a Study Session Leader for the Cal Poly Academic Success Center, is the treasurer of the Microsystems Technology Club (MST), and is the co-lead for the Composites subsystem of Cal Poly Racing Baja SAE.
Faculty Advisor: Dr. Jean Lee
Associate Professor, Materials Engineering
1 Grand Avenue
Cal Poly
San Luis Obispo, CA 93407
(805) 756-6571
jlee473@calpoly.edu
Acknowledgements
We would like to thank our faculty advisor Dr. Jean Lee (Materials Engineering), Dr. Amro El Badawy and Sarah Stabler (Environmental Engineering), Dr. Marie Yeung and Jennifer Vanderkelen (Biology), Tom Featherstone (Chemistry), Kevin Coulombe (Physics), Melonee Cruse (EH&S), Eric Beaton (Materials Engineering), our sponsors at the Office of Student Research, and the 2021 CENG SURP coordinators for their continuous support over the course of this project. We are grateful for the use of the labs in the Environmental Engineering Department, the Spectrophotometry Lab in the Biology Department, the FTIR Lab in the Chemistry Department, and the Metallography and FTIR Labs in the Materials Engineering Department in conducting this project.
OUR PROJECT
Abstract
Colorimetric sensing of pathogens in water is based on an enzymatic reaction between β-galactosidase (β-gal) and chlorophenol red-β-D-galactosidase (CPRG) that results in a color change from yellow to red (Figure 1). Previous researchers have added cationic gold nanoparticles (NPs) to bind to the negatively-charged β-gal, reducing the amount of β-gal available to react with CPRG and lowering the intensity of the red color¹. When negatively-charged pathogens such as bacteria are present in the water, they are attracted to the cationic gold NPs. This frees up the β-gal that was bound to the NPs to react with CPRG, resulting in an increase in the intensity of the red color that is proportional to the pathogen concentration. In this project, it is hypothesized that the interaction between the enzyme and cationic material is the result of Coulombic forces rather than a chemical reaction. Therefore, the attraction of negatively-charged pathogens to cationic materials should free up β-gal to react with CPRG and cause a color change. This project seeks to identify low cost, non-toxic materials that can perform the same function as the more expensive gold NPs.
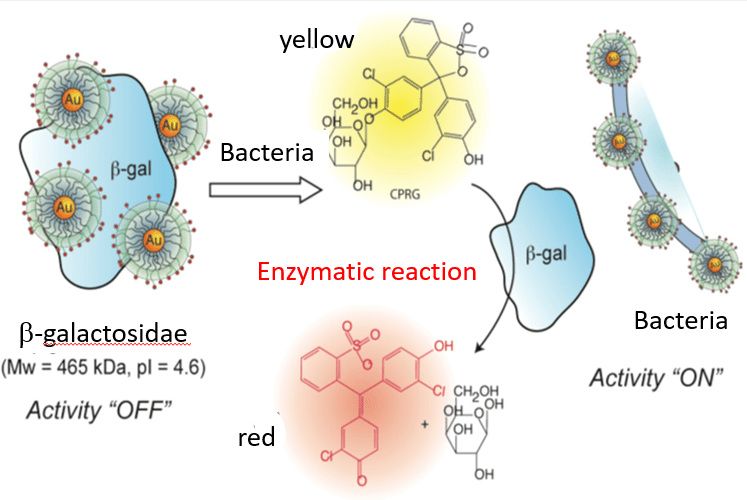
Figure 1: Interaction of β-gal and CPRG as a colorimetric sensor¹
Materials and Methods
Several positively-charged materials were examined to test the hypothesis, and were chosen based on charge, toxicity, and cost. Many of these candidate materials are non-toxic nanoparticles (NPs) in an aqueous solution; these NPs were tested by diluting the as-received material with de-ionized water or by coating with a cationic polymer.
Nanoparticles (NP):
All NPs were in aqueous solutions and were diluted to concentrations between 200 µM to 2000 µM based on treatment method. Treatment method refers to the cationic polymer used to coat the NPs.
Figures in parentheses are NP diameters.
- SiO2 (30 nm)
- TiO2 (30-50 nm)
- Fe3O4 (10 nm)
- CeO2 (30-50 nm)
- Al2O3 (30 nm)
- CuO (25-55 nm)
- ZnO (30-40 nm)
- MgO (50 nm)
Cationic Polymers Used to Coat NPs:
Coated NPs were coated with only one of the cationic polymers. Each polymer is considered a separate treatment. Different procedures were used for each treatment.
- 2500 MW linear polyethylenimine (PEI)
- 1200 MW branched PEI (BPEI)
- 20% aqueous solution polyhexamethylene biguanide hydrochloride (PHMB)
The following procedure was used to functionalize NPs with PEI or BPEI:
- Combine equal volumes of 20 mg/mL PEI and 3 mg/mL NP
- Sonicate for 2 minutes
- Place in shaking incubator at room temperature overnight at 100 RPM
- Centrifuge 3 times for 2 minutes: Twice at 3000 RPM, once at 2000 RPM
- Sonicate for 2 minutes
The following procedure was used to functionalize NPs with PHMB:
- Combine 50 μg/mL PHMB and 2000 μM NP
- Mix on stir plate for 2 hours
- Sonicate for 2 minutes
- Store in refrigerator until ready to use
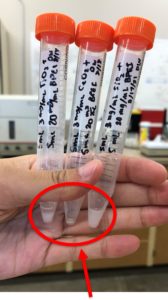
Figure 2: BPEI-coated NPs

Cal Poly Office of Student Research
Materials and Methods (cont'd)
Several bulk candidate materials were also tested due to their charge or ability to be charged and easily retrieved.
Bulk Materials:
- Positively-charged nylon membrane (woven nylon fibers derivatized with quaternary ammonium groups)
- p-type silicon wafer pieces
- Buff sponge (polyester)
- Cosmetic wedge (styrene butadiene and/or nitrile butadiene synthetic foam)

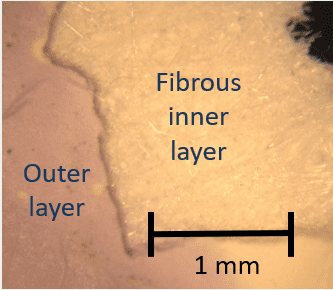
Figure 3: Buff sponge (left) and cosmetic sponge (right).
Figure 4: Dark field optical micrograph showing layers of the nylon membrane.
Preparation of wells in the plate followed the general procedure outlined below, where β-gal, CPRG, and the candidate material were added to phosphate buffer solution (PBS, pH ~7) in each well. Control wells contained only PBS, β-gal, and CPRG.
- Add 100 μL PBS to well (130 μL in control wells)
- Add 30 μL β-gal to well
- Add 30 μL candidate material and mix
- Add 90 μL CPRG after 1 hour and mix
The addition of CPRG gives a complete colorimetric response within 2 hours, measured with a spectrophotometer (SP). If the candidate material successfully inhibits enzymatic activity, the well will remain the yellow color of the CPRG (positive result). If the candidate material doesn’t inhibit enzymatic activity, the well will gradually change to a reddish color (negative result) as shown in Figure 5.
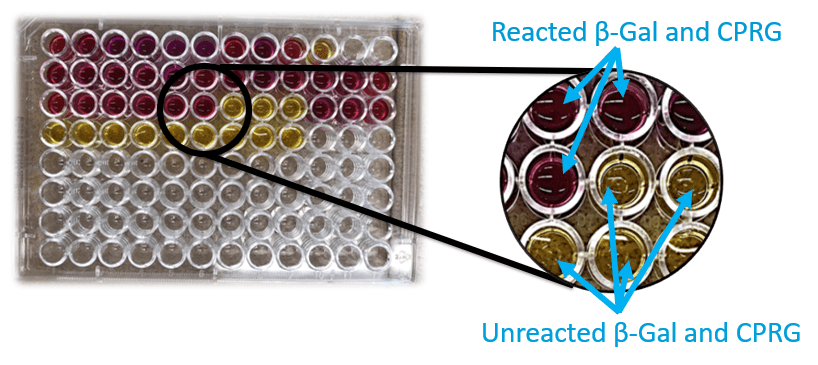
Figure 5: Colorimetric assay of candidate materials using a 96-well plate.
Discussion of Results
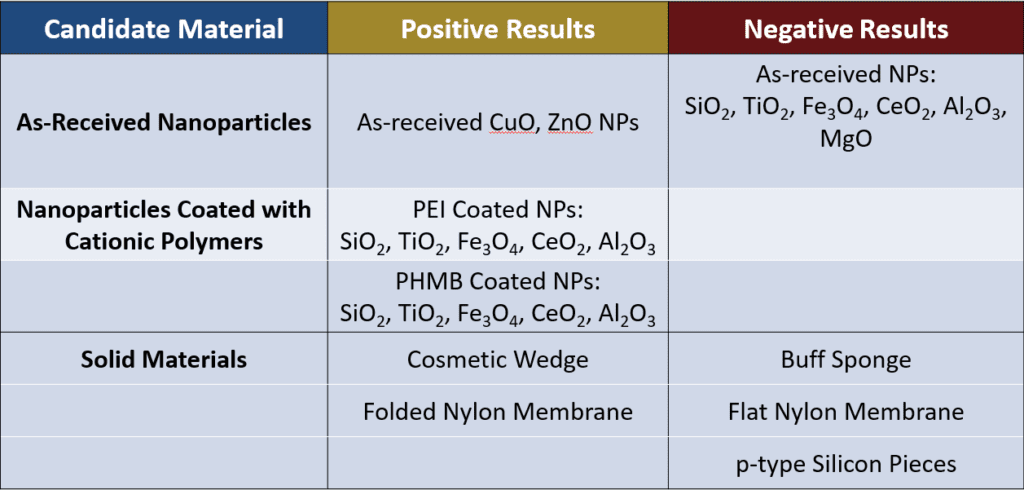
Several trends are evident in the results:
- Among the as-received NPs, only CuO and ZnO NPs gave positive results. Both the CuO and ZnO NPs have high isoelectric points (IEPs) of 9.5 and 8.7-10.3 respectively, so in PBS (which has a pH similar to water), these NPs have a relatively positive charge. MgO also has a high IEP (around 12) but gave a negative result when tested. Further testing with MgO gave inconclusive results.
- NPs with low IEPs with respect to PBS (and water) tended to give negative results.
- The addition of cationic polymers to NPs indicated positive results. Given the limitations of the equipment used, it was difficult to ascertain if the positive results were due to the presence of the cationic polymer rather than the polymer coating the NPs (Figure 4).
- The cosmetic wedge and the folded nylon membrane indicated successful inhibition of enzymatic activity but appeared to absorb some of the solution when removed from the wells, leading to questions about the effect of material geometry on the results.
Effect of Geometry
An unexpected result appeared in some of the bulk materials tested: despite a positive charge, the bulk materials varied in effectiveness based on their geometry.
- Before testing, the buff sponge was very fibrous and had a low density, while the cosmetic wedge was porous and relatively denser. When removed from their wells, the buff sponge was unchanged while the cosmetic wedge was swollen and yellow in color.
- The positively-charged nylon membrane was tested in different folded configurations to investigate the effect of geometry. When removed from the well, the membranes had purple edges and creases where the inner layer of the membranes were exposed to the liquid. The areas where the inner layer of the membranes was most protected from the liquid were pale yellow (Figures 6a and 6b).
- The p-type Si pieces may not have had enough surface area to bind with enough β-gal and thus did not significantly inhibit enzymatic behavior.
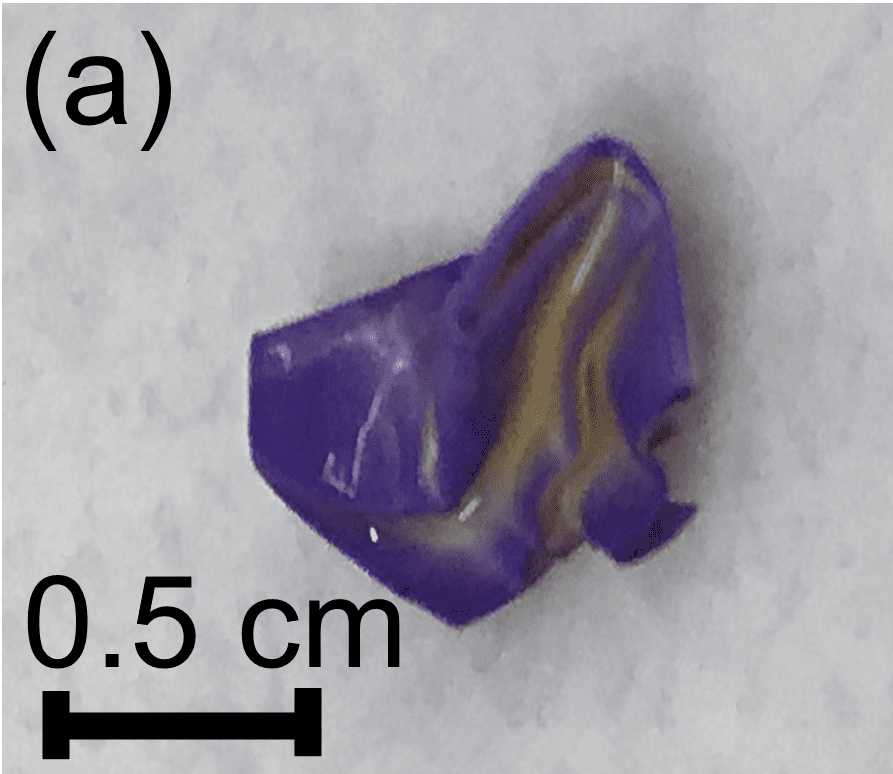
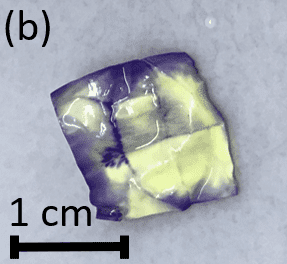
Figure 6: (a) Folded nylon membrane with a purple color on the surfaces exposed to the liquid, (b) Unfolded nylon membrane with color differences due to exposure to the liquid.
Conclusions
Initial results from our proof-of-concept experiments show promise in identifying new non-toxic and inexpensive candidate materials for colorimetric water pathogen detection. For nanoscale candidate materials, positive results from high IEP NPs is consistent with our hypothesis that the interaction of the materials with β-gal is Coulombic rather than chemical in nature. However, when considering bulk materials for use as colorimetric sensors, the geometry of the material needs to be taken into consideration.
Next steps should focus on validating results with non-pathogenic E. coli; exploring the recoverability and reuse of promising candidate materials; and understanding the unusual results from MgO NPs, the nylon membrane, and the cosmetic wedge.
References
(1)Miranda, O. R. et al., J. Am. Chem. Soc. 2011, 133:9650-9653.
(2)Thiramanas, R. and Laocharoensuk, R., Microchimica Acta. 2016, 183:389-396.
(3)Ashraf S. et al., Nanoscale Res Lett. 2012, 7(1):267.